Clinical Data Management
Advantages of CORE OBJECTIVE




Our Clinical Data Management Services
Right combination of onshore and offshore services is used to pass on the financial benefits to our clients.
Along with the CDM activities we have a dedicated team of Doctors, Pharmacists, Nurses, Physiotherapists, Paramedical Staff and associated resources to provide actual research Execution, Monitoring and project management activities for conduction of Phase II, III and IV trials in USA, UK, India and other places in the world.
Our Key Team members and Board Members are HCPs, Healthcare IT professionals for many years. They have strong connections with US, UK and India based networks of hospitals. These connects can help CROs, Clinical Trial Management organizations and Pharma companies in faster patient recruitment for the Clinical Trials.
Additionally, highly qualified Investigators can be made available to conduct the trials efficiently and ethically.
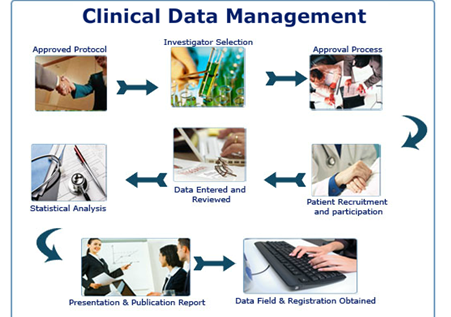
1. Study Startup
- CRF Annotation
- Data management plan development
- CRF/eCRF design- Preparing GUI for the CRF pages. This can be done both as standalone application and RDC (Remote Data Capture) depending on the client specification and budget.
- Preparing application to pull data from the GUI and place in the respective field of the tables in the database.
- Design the tables and schema in the database.
- Database validation
3. Study Closeout
- Final database closure activities.
- Database lock.
- Project document filing
2. Study Conduct
- Customize and Develop Web/ Mobile application for Electronic Data Capture (EDC)
- Prepare integrated application to import data directly from the labs into the database to avoid manual errors while loading the data.
- Prepare backup strategies for the database.
- Help client in data upload if PDF’s of CRF are sent (offshore activity to save on budget).
- Double data entry and verification
- Data loading(Central Lab Data, e-patient Diary, PK data Loading)
- Data review and Query Management
- Clinical Data Coding, Project specific document management
- Keep track of Adverse Events and Serious Adverse Events (if demanded prepare a different database for AE and SAE as per FDA norms).
- Automatic generation of DCF to the site in case of data discrepancy.
- Analyze and audit the database at frequent intervals (Quality control and assurance methodologies used).
- Generate queries needed by the client for data analysis by Statisticians.
- Give offshore services to the client in order to save money.
Statistical Analysis

We provide the following statistics services:
- Protocol and CRF Development
- Randomization schedules
- Sample size calculations
- Statistical consultancy
- Statistical analysis
- Analysis, tables, figures and listings programming
- Statistical reporting
- Input into the integrated clinical/statistical reports
High Quality, Security and Cost Efficiency
Accurate
Accessible
Reproducible
Source-verified
Timely and cost-efficient
Clinical Data Management is broken into very specific key tasks and all of them need to be managed by a Lead Data Manager. This individual will be the single point of contact for all your Clinical Data Management needs. We have defined metrics and turnaround times for each step of our processes from database build and entry to query resolution and QC. This allows us to efficiently manage our time and ensure we provide you with accurate costs at the beginning of any study, which minimizes change orders during the study.
We have a standard set of reports available that will detail each aspect of the data processing. These can be sent as regularly as required to allow productive meetings, especially towards database lock. Our dedicated CRF design team use latest tools and methods to create Case Report Forms, Source documentation, Diaries and Urinalysis booklets. Each CRF is based on our RPL standard library of CRF modules . However our team will modify or create CRFs in accordance with your needs to ensure you receive the module you specifically require. CRF instructions are prepared alongside the design of the CRF to ensure site staffs are very clear on how to complete the required pages. These can be incorporated into the document itself or be a standalone document.
CORE OBJECTIVE offers CDM services with maximum flexibility. Sponsors may contract all or a portion of their CDM work load to CORE. Our infrastructure and clearly defined system interfaces provide us the flexibility to define work processes between a sponsor system and our environment as well as seamless integration in to sponsor defined processes. Our systems & services are based on industry recognized standards and technology platforms to ensure that even the most challenging data management activities are efficiently and effectively addressed.
Coding specialists perform the coding in the standard and proprietary coding dictionaries Discrepancy handling, Laboratory data handling and Data extract.
Our Differentiates in Clinical Data Management
- Qualified Database Programmers experienced in designing and implementing Clinical trial databases and data validation programs.
- Coding specialists to assist coding in standard and proprietary coding dictionaries such as WHODD, and ICD-9-CM.
- Specialists experienced in drafting CRFs and questionnaires.
- Trained and experienced data entry specialists.







