Clinical Trials Project Management
Clinical Trials Management
CORE OBJECTIVE’s SMO (Site Management Organization) provides medical institution with several supporting services to Informed Consent, preparation of case report form. This service promotes clinical trial implementation smoothly and efficiently at each phase of clinical development.
Clinical Trial Site And Project Management activities for Sites include but are not limited to following activities:

- Site Identification
- Site selection
- Site inspection
- Investigator identification and selection
- Resource Management
- Site monitoring
- Patient recruitment and follow-up
- Drug management – end to end
- Data management
- Documentation
- Regulatory affairs compliance
- IRB coordination
- Site closure
End to End Project Management can be done with expert people already working in this field. Some of the experienced staff of Core Clinical’s are already in place, working on different aspects of health care field.
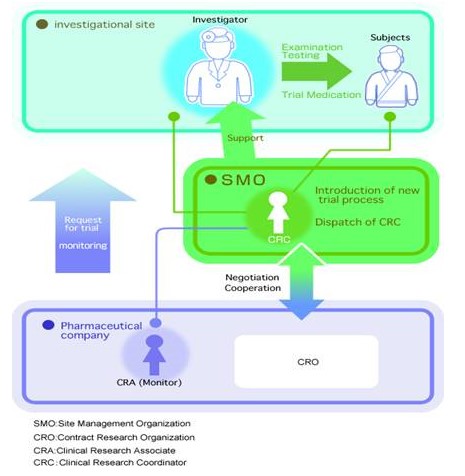
Position of SMO within the clinical trial process
We act as a bridge between medical institution and pharmaceutical companies at the front lines of clinical development. This will make a large contribution to the smooth implementation of clinical trial from site selection to preparation of clinical study report.
CORE’s SMO (Site Management Organization) provides medical institution with several supporting services to Informed Consent, preparation of case report form. This service promotes clinical trial implementation smoothly and efficiently at each phase of clinical development.







